FDA enforces recall of hypertension drugs over cancer-causing impurities
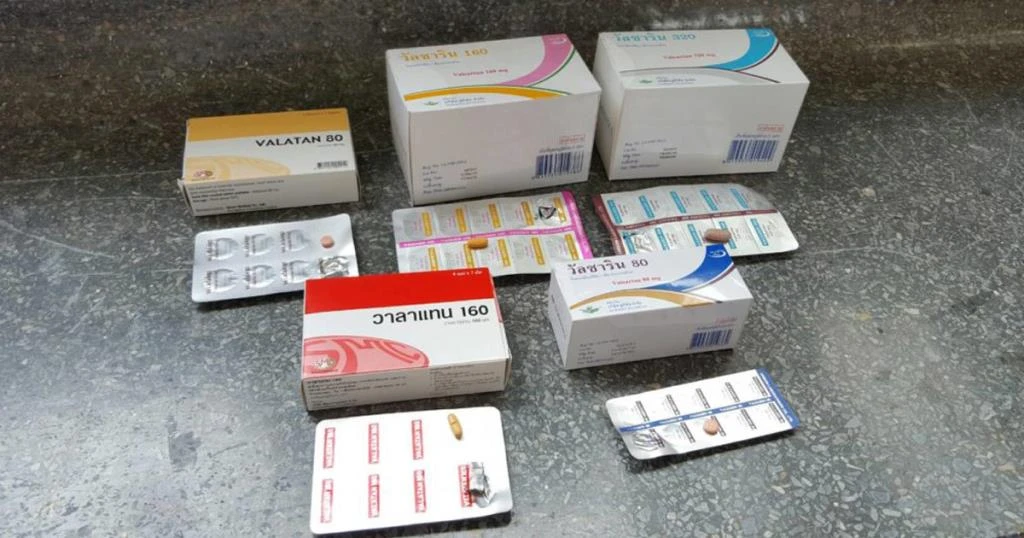
The FDA recalls 42 hypertension drug batches for cancer-causing impurities and advises vigilant and consistent medication use. The Food and Drug Administration (FDA) has recalled 42 batches of hypertension drugs due to potentially carcinogenic impurities found in some models of irbesartan. The impurities were detected after the detection of azidomethyl biphenyl tetrazole (AZBT) impurities in certain models of the drug. The FDA has issued a recall order for the affected batches from hospitals, clinics, pharmacies, and drug manufacturers. Despite the recall, the FDA has assured that other FDA-approved models remain safe for consumption. The recall was prompted by an examination of samples collected from every manufacturer.
Diterbitkan : 2 tahun lalu oleh Full Stack Web Developer, https://github.com/demirdoven, Mitch Connor, [email protected], Selman Demirdoven, [email protected] di dalam Health
A recall of 42 batches of hypertension drugs has been enforced by the Food and Drug Administration (FDA) from five pharmaceutical manufacturers following the discovery of potentially carcinogenic impurities. The decision came after the detection of azidomethyl biphenyl tetrazole (AZBT) impurities in some models of irbesartan, a drug used to control high blood pressure.
Acting secretary-general of the FDA, Narong Aphikulvanich has confirmed the recall, citing increased cancer risk for patients as the primary reason. Despite the recall of certain models, Narong assures that other FDA-approved models of irbesartan remain safe for consumption.
In response to the contamination, the FDA has instructed manufacturers of irbesartan to conduct thorough inspections for AZBT impurities. They are also required to replace contaminated ingredients with non-AZBT alternatives and to revisit their market distribution of the drug, reported Bangkok Post.
The FDA’s call to action was prompted by the examination of irbesartan samples collected from every manufacturer. Subsequent analysis by the Department of Medical Sciences revealed that certain models of the drug contained impurities at levels exceeding international safety standards, thereby increasing cancer risks.
As part of the corrective measures, the FDA has issued an order for the withdrawal of the affected irbesartan batches from hospitals, clinics, pharmacies, and drug manufacturers. However, Narong advised patients against abruptly discontinuing their use of irbesartan, given the importance of consistent medication for managing hypertension. Instead, patients are urged to inspect their medication vigilantly.
The five pharmaceutical companies implicated in the distribution of the nine contaminated irbesartan batches are TO Chemicals Co Ltd, Siam Bheasach Co Ltd, M&H Manufacturing Co Ltd, the Government Pharmaceutical Organisation, and Sriprasit Pharmacy Co Ltd.
In May, the Thailand Food and Drug Administration (FDA) uncovered a shocking revelation regarding a herbal drink under The Phi (เทพี) brand, which translates to “goddess” in Thai. The drink, known for its exaggerated claims of curing various ailments such as allergies, disk herniation, and rheumatoid arthritis, was found to contain steroids by the FDA. To read more click HERE
Topik: FDA
