Effectiveness of implementing a locally-developed guideline for antibiotic treatment of lower urinary tract infection in adults in Thailand - Scientific Reports
Lower urinary tract infection (UTI) is still a major concern in clinical practice, but inappropriate antibiotics are commonly prescribed in Thailand. This study aimed to develop, implement, and evaluate the effectiveness of a clinical practice guideline (CPG) for antibiotic treatment of lower UTI in adults at Siriraj Hospital which is a university hospital in Thailand. This study comprised a retrospective cohort study development phase, and a prospective cohort study implementation phase. The outcomes of treatment were compared between phases. The development and implementation phases enrolled 220 and 151 patients, respectively. The CPG compliance rate was significantly increased from 17.3% during the development phase to 43.0% during the implementation phase (p = 0.001). The rates of fluoroquinolones and cotrimoxazole use were significantly lower during implementation than during development (p < 0.001 and p = 0.027, respectively). The rates of nitrofurantoin and fosfomycin use were significantly greater during implementation than during development (p = 0.009 and p = 0.005, respectively). The overall cure rate was not significantly different between the two study phases, but implementation group patients had significantly more unfavorable prognostic factors than development phase patients. CPG-compliance group patients had a significantly higher cure rate than CPG-non-compliance group patients (p = 0.011). The cost of the initial course of antibiotics per episode was significantly higher during the implementation phase because the cost of fosfomycin is more expensive and more fosfomycin was prescribed during implementation (p = 0.047). Implementation of the locally-developed CPG was found to be effective for increasing the appropriate use of empirical antibiotics and increasing the cure rate; however, measures to improve and reinforce CPG compliance are needed. The Thai government has approved a guideline for antibiotic treatment of lower urinary tract infection in adults in adults. The study was conducted at Siriraj Hospital, Mahidol University, Bangkok, Thailand. The requirement of written informed consent was waived due to the study being a quality of care improvement study that obtained data via retrospective medical chart review. The CPG was developed for general use among all outpatient departments that treat lower UTI. The effectiveness of this CPG, which was determined after 10 weeks of its implementation, was determined following the implementation phase data collected during 15 November 2021. To be eligible for inclusion in the study, eligible patients had to be aged 18 or older with a diagnosis or suspected to be caused by bacteria.
Diterbitkan : 2 tahun lalu oleh Tropical Medicine, School of Medicine, Rujipas, Thailand, Department of Medicine, Mahidol University, Faculty of Medicine Siriraj Hospital, Bangkok, Nakornratchasima, Thamlikitkul, Institute of Medicine, Pruettichai, Sirijatuphat, Wisutep, Suranaree University of Technologgy, Division of Infectious Diseases, Visanu di dalam Health
The protocol for this study was approved by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (COA no. Si 1029/2020). The requirement of written informed consent was waived by the Siriraj Institutional Review Board of the Faculty of Medicine Siriraj Hospital due to this being a quality of care improvement study that obtained data via retrospective medical chart review, and patient anonymity was fully preserved for all study patients. All study methods were carried out in accordance with relevant guidelines and regulations.
This study was conducted at Siriraj Hospital, which is a 2300-bed tertiary-care university hospital, where patients with UTI can attend many different outpatient departments.
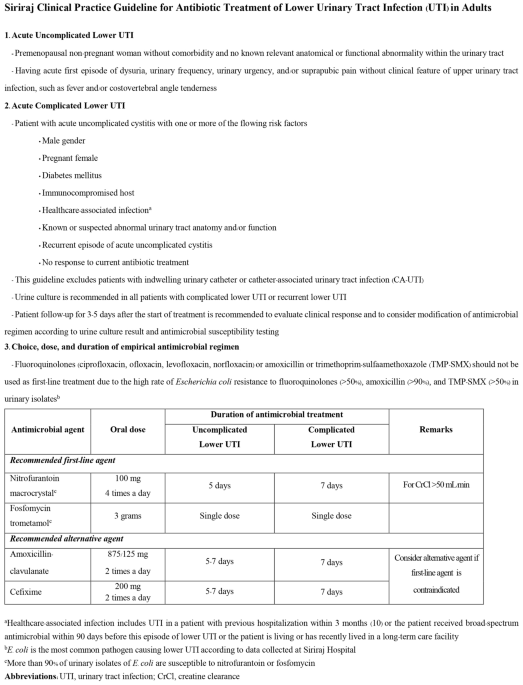
The study had two phases. The CPG development phase was an observational retrospective cohort study that reviewed the medical records of patients with UTI who attended Siriraj Hospital during 1 July 2020 to 30 April 2021 with diagnosis codes according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) of N300-N309 and N390. The collected information included demographics, clinical features, causative pathogens, antibiotic treatment and treatment outcomes of patients with acute lower UTI (both uncomplicated and complicated cases) without manifestation of sepsis or acute complication of UTI who were managed as ambulatory patients. The aforementioned information combined with the recommendations of the Infectious Disease Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESMID)2, the European Association Urology guidelines on urological infections11, and the local antibiogram of urinary isolates from the Global Antimicrobial Resistance Surveillance System at Siriraj Hospital6 and the Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University were used to develop the CPG for antibiotic treatment of lower UTI in adults with lower UTI at our center. The developed CPG is a simple and easy to use 2-page Thai language document (an English language translation is shown in Fig. 1). The contents of the CPG include the definition, diagnosis, investigation, and recommended antibiotic treatment of uncomplicated and complicated lower UTI in adults. This CPG is endorsed by the Division of Infectious Diseases and Tropical Medicine of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
The CPG implementation phase was a prospective cohort study to examine the effectiveness of the implementation of our locally-developed CPG for antibiotic treatment of lower UTI in adults at Siriraj Hospital. The CPG was developed for general use among all outpatient departments that treat lower UTI at our center. We intended to start the CPG implementation phase on 1 May 2021, but the implementation phase was conducted during 1 September 2021 to 14 November 2021 due to COVID-19-related complications/restrictions. At the start, CPG implementation was performed by using multifaceted interventions including posting the CPG in outpatient care areas; disseminating the CPG as brochures, circular letters, and social media to relevant physicians; organizing conferences on the use the CPG for antibiotic treatment of lower UTI in adults; and, introducing the CPG to the relevant outpatient departments via interactive two-way communication with the responsible physicians. Our infectious disease team visited the outpatient department before the office hour started for meetings with the responsible physicians and introduced the CPG to them day by day in the weekday. Meanwhile, we made an appointment with the relevant departments to organize a brief interactive lecture. After completing the aforementioned activity, a monthly meeting with each affected outpatient department was established to remind them about and reinforce the importance of strict compliance with the CPG.
After CPG implementation, we began to collect data of patients who met the same inclusion criteria as those enrolled during the CPG development phase. The effectiveness of this CPG was determined after 10 weeks of its implementation. Implementation phase data were collected during 15 November 2021 to 28 February 2022. The study flow diagram is shown in Fig. 2. In the case of missing clinical outcome data, patients were contacted by telephone to obtain the missing clinical outcome information.
To be eligible for inclusion, patients had to be aged 18 years or older with a diagnosis of lower UTI that was proven or suspected to be caused by bacteria, and that was treated at an outpatient department of Siriraj Hospital. We excluded patients with lower urinary tract symptoms that were proven or suspected to be caused by non-bacteria (e.g., radiation cystitis, chemotherapy-induced cystitis), patients with indwelling urinary catheter, patients who had received hematologic or solid organ transplantation, severely immunocompromised patients, and patients without available clinical outcome data by medical record review or phone call.
No previous study in Thailand has investigated the rate of appropriate use of antibiotic for adult ambulatory patients with lower UTI. It was estimated that a sample size of 200 patients was required for the CPG development phase based on the assumption that the rate of appropriate use of antibiotic in adult ambulatory patients with lower UTI prior to implementing the use of a locally-developed CPG was 30 ± 7% with a 5% type 1 error (two-sided) and a 10% increase to compensate for cases with missing data.
We estimated the appropriateness of antimicrobial treatment for lower UTI at Siriraj Hospital (CPG compliance group) to be 30% during the CPG development phase, and that this value would increase to 50% during the CPG implementation phase. To compare the proportion for independent two groups with a power of 80% and alpha error of 5%, the sample size of patients needed for each phase with an addition of 10% in case of data missing was at least 100 patients per phase with a total of at least 200 patients in both phases.
In summary, we need at least 200 patients for retrospective cohort study for the CPG development phase and a sample size and at least 100 patients for the prospective cohort study for the CPG implementation phase.
Urinary tract infection (UTI) was defined as the presentation of lower urinary tract symptom (s) caused by bacteria involving any part of the urinary tract confirmed by physician decision and/or evidence of pyuria (centrifuged urine leukocytes > 5 cells per high-power field).
Lower urinary tract infection was defined as a UTI of the urinary bladder and/or urethra, including cystitis and/or urethritis, without clinically suspected infection of the upper urinary tract.
Upper urinary tract infection was defined as a UTI of the kidney and/or ureter, including pyelonephritis and/or ureteritis, with usual clinical features, such as fever, costovertebral angle tenderness, and/or sepsis.
Uncomplicated UTI was defined as a UTI in a premenopausal woman (age ≤ 50 years) or a nonpregnant woman without comorbidities and no known relevant anatomical or functional abnormalities of the urinary tract.
Complicated UTI was defined as a UTI that did not satisfy the definition for uncomplicated UTI, including UTI in a male or pregnant woman, diabetes mellitus, immunocompromised host, healthcare setting, known or suspected abnormal urinary tract anatomy and/or function, and/or recurrent episode of acute uncomplicated cystitis within 3 months after the first episode of UTI.
Immunocompromised host was defined as a patient with neutropenia (absolute neutrophil count < 500 cells/mm3), immunosuppressive drug use, or acquired immunodeficiency syndrome (AIDS) with CD4 < 200 cells/mm3.
Healthcare-associated infections included a patient with previous hospitalization or who received broad-spectrum antimicrobial within 90 days before the current visit or who currently lives/recently lived in a long-term care facility.
Clinical practice guideline (CPG) compliance was defined as concordance between the antibiotic regimen prescribed for adult patients with a lower UTI and the antibiotic regimen recommended in the CPG.
Clinical practice guideline (CPG) non-compliance was defined as discordance between the antibiotic regimen prescribed for adult patients with a lower UTI and the antibiotic regimen recommended in the CPG.
Treatment outcomes were defined, as follows: (1) cure—resolution of all clinical features of UTI; (2) persistence—persistence of clinical features of UTI after complete treatment; (3) recurrence—occurrence of UTI within 30 days after the patient responded well to the given treatment; (4) misdiagnosis—definite diagnosis was not a UTI; and, (5) complication of UTI—progression of infection, including sepsis, upper UTI, epididymo-orchitis, requirement for hospitalization, or UTI-related death.
Due to the range of UTI antimicrobial treatment regimens, inappropriate choice, dose, or duration of treatment is common13. Accordingly, the establishment of and compliance with a CPG will reduce the inappropriateness of antimicrobial decision-making. The primary outcome of study was the rate of CPG compliance among the relevant physicians compared between the CPG development phase and the CPG implementation phase. The secondary outcomes were pattern of antibiotic use, outcomes of treatment, outcomes of treatment between the CPG compliance patients and the CPG non-compliance patients, and cost of treatment for adult patients with lower UTI compared between the CPG development phase and the CPG implementation phase.
Descriptive statistics were used to summarize and report patient characteristics. Comparisons of continuous data between groups were performed using unpaired Student t-test for normally distributed continuous data (findings reported as mean plus/minus standard deviation), and Mann–Whitney U test for non-normally distributed continuous data (findings reported as median and range). Comparisons of categorical data between groups were performed using chi-square test (for large sample size) or Fisher’s exact test (for small sample size) (findings reported as number and percentage). The data were analyzed using SPSS Statistics version 18.0 (SPSS, Inc, Chicago, IL, USA), A p-value of less than 0.05 was considered statistically significant for all tests.
The protocol for this study was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (COA no. Si 1029/2020). Written informed consent was not obtained from any patients in either the retrospective or prospective arms of this study because this was a quality of care improvement study that obtained data via medical chart review, and patient anonymity was fully preserved in all cases in both study phases.
Topik: Drugs, Thailand
